First Patient Enrolled in Phase II Clinical Trial of DECISION’s COMBAT Trial

• First patient enrolled at Hôpital Beaujon, Clichy, France
• The COMBAT Trial is a multicenter, randomized, open-label, Phase II clinical trial in patients with decompensated cirrhosis
Barcelona, Spain, 9th July 2024 – DECISION, an EU-funded research consortium in liver disease research, is pleased to announce the enrollment of the first patient at Hôpital Beaujon, Clichy, France in the COMBAT Trial, a multicenter, randomized, open-label, Phase II clinical trial. This trial is a crucial component of the DECISION project, which aims to transform the treatment of decompensated cirrhosis and acute-on-chronic liver failure (ACLF).
The COMBAT Trial, officially titled “A Novel COMBinATorial Therapy With Albumin and Enoxaparin in Patients With Decompensated Cirrhosis at High-risk of Poor Outcome,” seeks to determine the safety, efficacy, and cost-effectiveness of combining human albumin and ednoxaparin with standard medical treatment. Patients in the study are divided into two cohorts: one receiving the combinatorial therapy plus standard treatment, and the other receiving the best standard-of-care treatment for decompensated cirrhosis alone.
Prof. Dr. Paolo Caraceni, Principal Investigator of the COMBAT Trial and professor of Internal Medicine at the University of Bologna, Italy, stated, “We are excited to begin the COMBAT Trial and are hopeful that the combination of albumin and enoxaparin will offer a new therapeutic option for patients with decompensated cirrhosis. Our ultimate goal is to reduce mortality and improve quality of life for these patients.”
Prof. Dr. Pierre-Emmanuel Rautou, scientific coordinator of DECISION and professor of medicine at Hôpital Beaujon, Clichy, France added, “The initiation of the COMBAT Trial is a testament to the hard work and dedication of our researchers and partners. We are grateful to the patients who are participating in this study and look forward to advancing our understanding of albumin and enoxaparin through this clinical trial.”
The COMBAT Trial is conducted by work package 5 (WP5) of the DECISION project, ensuring rigorous clinical oversight and a patient-centric approach. Participants will attend study visits to monitor disease progression and response to the therapy, providing critical data to evaluate the trial’s objectives. This trial is funded by the European Union under Horizon 2020 framework, reflecting the importance of innovative research in addressing unmet medical needs.
About DECISION
The DECISION project (www.decision-for-liver.eu) aims to transform the treatment of decompensated cirrhosis and acute-on-chronic liver failure (ACLF) by leveraging systems-level understanding of their pathophysiology. Utilizing extensive, well-characterized patient cohorts, DECISION seeks to significantly reduce mortality with personalized combinatorial therapies tailored to individual patient needs.
Commencing on April 1, 2020, and spanning 66 months, the project involves 21 institutions from 10 European countries with 6 million euros in EU funding. Led by Prof. Pierre-Emmanuel Rautou (EF CLIF), DECISION focuses on developing a prognostic test to identify high-risk patients and a response test to predict success with novel combinatorial therapies.
For more information, visit www.decision-for-liver.eu and follow us on X (formerly known as Twitter) and LinkedIn.
About the COMBAT Trial:
The COMBAT Trial is a pioneering clinical study designed to evaluate the safety, efficacy, and cost-effectiveness of a novel combinatorial therapy involving human albumin and enoxaparin for patients with decompensated cirrhosis. Officially titled “A Novel COMBinATorial Therapy With Albumin and Enoxaparin in Patients With Decompensated Cirrhosis at High-risk of Poor Outcome (COMBAT Trial),” this Phase II randomized controlled trial is a key component of the DECISION project.
The primary goals of the COMBAT Trial are to determine:
1. Safety and Tolerability: Is the combinatorial therapy safe for patients?
2. Efficacy: Is the combinatorial therapy effective in improving patient outcomes?
3. Cost Comparison: Does this therapy cost more or less than standard medical treatment?
Participants in the trial will attend study visits where various tests will be conducted to assess disease progression while they are taking the study medication. The study will compare an experimental group receiving the combinatorial therapy plus standard treatment with a control group receiving only standard treatment to evaluate differences in safety, efficacy, and cost.
Study Details:
• Study Contact: Anna Bosch (General Manager of EF CLIF)
Phone: +34 93 227 14 03
Email: anna.bosch@efclif.com
• Inclusion Criteria:
Ages 18-80 years.
Patients with decompensated cirrhosis admitted to the hospital due to acute decompensation (AD) as per EASL-CLIF criteria.
CLIF-C AD score >= 50 at admission or any time during hospital stay.
Recovery from AD and expected discharge within 48-72 hours.
Interventional Model Description:
• Cohort 1: Standard medical treatment plus a combinatorial therapy of enoxaparin and human albumin.
• Cohort 2 (Control): Standard medical treatment only.
Study Centers:
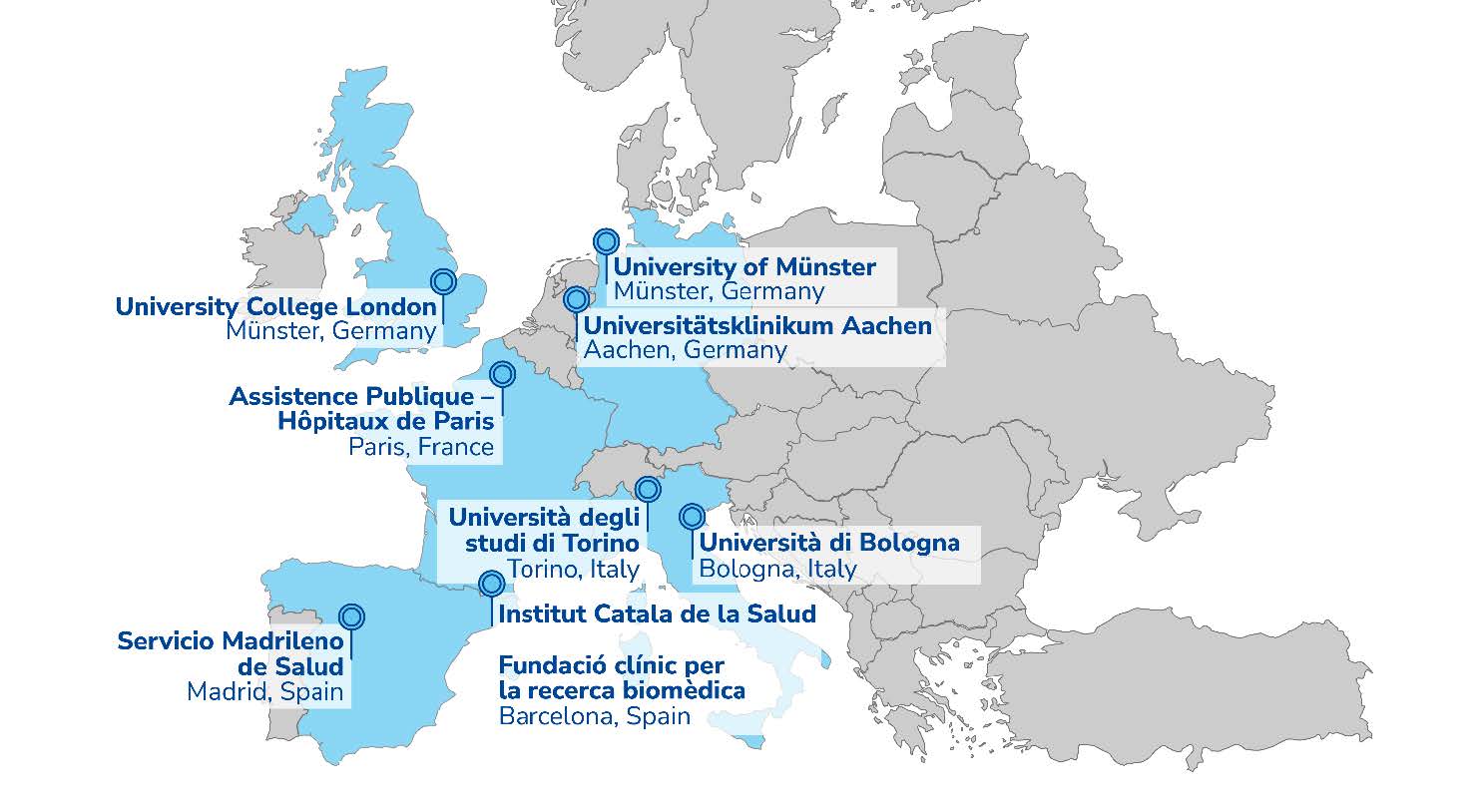
The COMBAT Trial is conducted by work package 5 (WP5) of the DECISION project. For more information, visit www.decision-for-liver.eu.
www.decision-for-liver.eu
Contact
Prof. Pierre-Emmanuel Rautou
DECISION Coordinator
pierre-emmanuel.rautou@inserm.fr
+ 33 (0) 1 40 87 52 83
Tamara Breitinger
Dissemination
tamara.breitinger@concentris.de
+49 (0) 8141 6252 8578
Sonja Leissner
Project Manager
sonja.leissner@concentris.de
+49 (0) 8141 6252 38588
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 847949. This press release reflects only the view of the author or authors (scientific coordinator and contact & translating personnel), and the European Commission is not responsible for any use that may be made of the information it contains. Reproduction is authorised provided the source is acknowledged.


