Antiviral therapy improves survival rates for kidney transplant recipients with hepatitis B or C
Amsterdam, March 14, 2019 – Prior to the development of antiviral therapy, kidney transplant recipients infected with either hepatitis B (HBV) or hepatitis C (HCV) experienced poor outcomes. In a new study in the Journal of Hepatology, published by Elsevier, researchers report favorable 10-year survival rates for patients with HBV and/or HCV treated with antiviral agents and advise that antiviral therapy should be systematically offered to all HBV and HCV patients in line with international recommendations.
Renal transplantation is currently the best treatment for patients with end-stage renal disease (ESRD) because it significantly improves survival compared to patients who remain on hemodialysis. Since the 1990s, antiviral therapy using nucleos(t)ide analogues like adefovir, lamivudine, tenofovir, and entecavir, has benefited HBV-infected patients by preventing viral replication in infected cells. Interferon-based therapy has been rarely used in HCV-infected patients because it increases the risk of transplant rejection. Although the prevalence of HBV and HCV infection in patients with ESRD has significantly declined over time, it remains at least four times higher than in the general population.
“With the improvement of HBV treatment in the last two decades and the development of new treatments against HCV, an update of data in large cohorts of kidney transplant recipients with long-term follow-up was warranted,” explained lead investigator Philippe Mathurin, MD, PhD, CHRU de Lille, Chief, Service des maladies de l’appareil digestif, Université Lille 2 and Inserm U795, Lille, France.
To reassess the impact of HBV and HCV on patient and kidney transplant graft survival, researchers analyzed records of more than 30,000 kidney transplant recipients using the French national database CRISTAL, including 575 recipients with HBV, 1,060 with HCV, and 29,798 who were free from infection. The first step of this study compared prognoses in these three groups and confirmed a poorer prognosis in HCV-infected patients than in non-infected patients. In contrast, there was no significant difference in patient and graft survival in HBV-infected patients compared with non-infected patients.
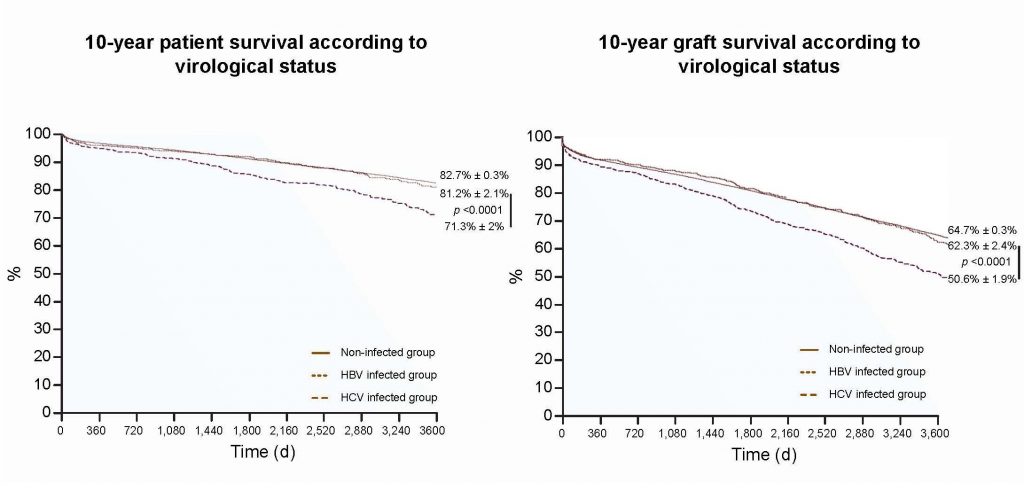
In the second step of this study, a random analysis of the medical records of 184 HBV and 504 HCV patients showed a control of viral replication in 94 percent and 35 percent of cases, respectively. For each kidney transplant recipient with HCV with detectable or undetectable HCV RNA, researchers randomly selected one to four matched controls from the 29,797 non-infected patients using the following matching criteria: gender, age, duration of dialysis, duration of cold ischemia, and year of transplantation. This analysis provided evidence that HBV chronic infection, which previously had a negative impact on patient and survival in kidney transplant patients, no longer influences patient or graft survival due to the control of viral replication related to the extensive use of nucleos(t)ide analogues. In contrast, HCV chronic infection still negatively impacts 10-year patient and graft survival. However, HCV’s negative influence is counteracted by sustained viral suppression, since it is no longer observed in kidney transplant recipients with undetectable HCV RNA.
“The control of viral replication is a key factor in kidney transplant recipients,” commented Dr. Mathurin. “The present results suggest, in line with international and KDIGO (Kidney Disease: Improving Global Outcomes) guidelines, that antiviral therapy should be systematically offered to HBV- and/or HCV-infected kidney transplant recipients or candidates to prevent the impact of chronic viral replication. Antiviral therapy with nucleos(t)ide analogues or direct antiviral agents should systematically be proposed, considering the major impact on patient and graft survival.”
—
Notes for editors
The article is “Control of replication of hepatitis B and C virus improves patient and graft survival in kidney transplantation,” by Hélène Fontaine, MD; Laurent Alric, MD, PhD; Julien Labreuche, PhD; Benjamin Legendre, PhD; Alexandre Louvet, MD, PhD; Corinne Antoine, MD; Christophe M. Legendre, MD, PhD; Marc Hazzan, MD; Nassim Kamar, MD, PhD; Sebastien Dharancy, MD, PhD; Stanislas Pol, MD, PhD; Alain Dhuamel, MD, PhD; and Philippe Mathurin, MD, PhD (http://dx.doi.org/10.1016/j.jhep.2018.12.036). It will appear in the Journal of Hepatology, volume 70, issue 5 (May 2019) published by Elsevier.
Full text of this article is available to credentialed journalists upon request; contact Carolien van der Zanden at +31 20 485 3346 or hmsmedia@elsevier.com. Journalists wishing to interview the study authors should contact Philippe Mathurin, MD, PhD, at + 33 6 80 06 43 18 or Philippe.MATHURIN@CHRU-LILLE.FR.
This study was supported by a grant from ANRS (Agence Française de Recherche sur le VIH/Sida et les Hépatites Virales) without any role.
About the Journal of Hepatology
The Journal of Hepatology is the official journal of the European Association for the Study of the Liver (EASL). It publishes original papers, reviews, case reports, and letters to the Editor concerned with clinical and basic research in the field of hepatology. www.journal-of-hepatology.eu
About EASL
In the fifty plus years since EASL was founded, it has grown from a small organization that played host to 70 participants at its first meeting, to becoming the leading liver association in Europe. EASL attracts the foremost hepatology experts as members and has an impressive track record in promoting research in liver disease, supporting wider education, and promoting changes in European liver policy. www.easl.eu
About Elsevier
Elsevier is a global information analytics business that helps scientists and clinicians to find new answers, reshape human knowledge, and tackle the most urgent human crises. For 140 years, we have partnered with the research world to curate and verify scientific knowledge. Today, we’re committed to bringing that rigor to a new generation of platforms. Elsevier provides digital solutions and tools in the areas of strategic research management, R&D performance, clinical decision support, and professional education; including ScienceDirect, Scopus, SciVal, ClinicalKey and Sherpath. Elsevier publishes over 2,500 digitized journals, including The Lancet and Cell, 39,000 e-book titles and many iconic reference works, including Gray’s Anatomy. Elsevier is part of RELX Group, a global provider of information and analytics for professionals and business customers across industries. www.elsevier.com

